 Cannabis And Arthritis
Cannabis And Arthritis
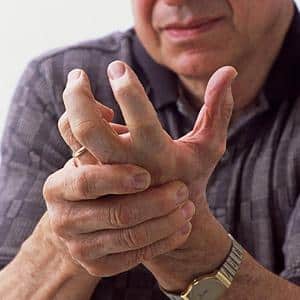
More than 31 million Americans suffer from arthritis. There are two common types of arthritis, rheumatoid arthritis and osteoarthritis, but both affect the joints, causing pain and swelling, and limiting movement.
Rheumatoid arthritis is caused by a malfunction of the immune system. Instead of fighting off intruders such as bacteria or viruses, the body attacks the synovial membranes, which facilitate the movement of joints, eventually destroying cartilage and eroding bones. Rheumatoid arthritis is most common among the aged, whose immune systems are no longer as robust or efficient. Osteoarthritis, or arthritis of the bones, is also found primarily among the elderly, where cartilage has been worn away through many years of use. Arthritis may also manifest as chronic inflammation of the joints as the result of injuries.
The use of cannabis as a treatment for musclo-skeletal pain in western medicine dates to the 1700s. Evidence from recent research suggests that cannabis-based therapies are effective in the treatment of arthritis and the other rheumatic and degenerative hip, joint and connective tissue disorders. Since these are frequently extremely painful conditions, the well-documented analgesic properties of cannabis make it useful in treating the pain associated with arthritis, both on its own and as an adjunct therapy that enhances the efficacy of opioid painkillers.
But cannabis has also been shown to have powerful immune-modulation and anti-inflammatory properties,suggesting that it could play a role in treating arthritis, and not just in symptom management. In fact, one of the earliest records of medical use of cannabis, a Chinese text dating from ca. 2000 BC, notes that cannabis “undoes rheumatism,” suggesting its anti-inflammatory effects were known even then.
Modern research on cannabidiol (CBD), one of the non-psychoactive components of cannabis, has found that it suppresses the immune response in mice and rats that is responsible for a disease resembling arthritis, protecting them from severe damage to their joints and markedly improving their condition.
Human studies have shown cannabis to be an effective treatment for rheumatoid arthritis, one of the many recognized conditions for which many states allow legal medical use. Cannabis has a demonstrated ability to improve mobility and reduce morning stiffness and inflammation. Research has also shown that patients are able to reduce their usage of potentially harmful Non-Steroidal Anti-Inflammatory drugs (NSAIDs) when using cannabis as an adjunct therapy.
Medical researchers at Hebrew University in Jerusalem found that when Cannabidiol is metabolized, one result is the creation of an acid with potent anti-inflammatory action comparable to the drug indomethacin, but without the considerable gastrointestinal side effects associated with that drug.
In addition, when the body metabolizes tetrahydrocannabinol (THC), one of cannabis’ primary components, it produces a number of related chemicals. At least one of these metabolites has anti-inflammatory and pain-relieving effects. By modifying this metabolite, researchers at the University of Massachusetts Medical Center have produced a synthetic carboxylic acid known as CT-3 (also called DMH-11C, chemical name dimethylheptyl-THC-11 oic acid), which is more powerful than the natural metabolite itself, and thus can be given in smaller doses. Animal tests found CT-3 effective against both chronic and acute inflammation, and it also prevented destruction of joint tissue from chronic inflammation. The long safety record of marijuana – no one has ever died of an overdose – and the fact that a metabolite with the desired anti-inflammatory effect is produced in the body when marijuana is used, strongly suggest that safe and effective anti-inflammatory drugs may be developed from cannabinoids.
In addition, CT3 has demonstrated analgesic effects in animals. In some cases the dose-dependent effect of THC was equivalent to morphine, but with a much greater duration of action.
In contrast to the NSAIDs commonly prescribed arthritis sufferers, CT3 did not cause ulcers at therapeutically relevant doses. Moreover, it does not depress respiration, exhibit dependence, induce body weight loss or cause mutations. Studies on its mechanism of action are currently underway, with cytokine synthesis one of the pathways being studied.
Cannabis may also help combat rheumatoid arthritis through its well-recognized immune-modulation properties. Rheumatoid arthritis is characterized by dysregulation of the immune system in response to an initial infection or trauma. Over-activity of the immune system’s B-cells causes antibodies to attack and destroy the synovial tissues located in the joint.
The immuno-modulatory properties of a group of fats found in cannabis known as sterols and sterolins have been used as natural alternatives to conventional rheumatoid arthritis treatments, which employ highly toxic drugs to either suppress the entire immune response of the body or to palliate pain and the inflammatory process without correcting the underlying immune dysfunction.
Cytokines play a role in either fueling or suppressing the inflammation that causes damage in rheumatoid arthritis and some other diseases. The release of selected cytokines is impaired by cannabis, but the findings differ by cell type, experimental conditions, and especially the concentration of the cannabinoids examined. A sterol/sterolin combination has been experimentally demonstrated to reduce the secretion of the pro-inflammatory cytokines controlled by the TH2 helper cells and to increase the number of TH helper cells that regulate the secretion of antibodies from the B cells. This selective activation and inhibition of the immune system results is an effective control of the dysfunctional auto-immune response.
Similarly, ajulemic acid (another non-psychoactive cannabinoid) has been found by UMass Medical Center researchers to reduce joint tissue damage in rats with adjuvant arthritis. Tests on human tissue done in vitro showed a 50% suppression of one of the body’s chemicals (interleukin-1beta) central to the progression of inflammation and joint tissue injury in patients with rheumatoid arthritis.
Conventional Arthritis Medications
Nearly 100 medications are listed by the Arthritis Foundation website for use with arthritis or other related conditions, such as fibromyalgia, psoriasis, osteoporosis and gout. These medicines include aspirin, ibuprofen and other oral and topical analgesics that dull pain. The most commonly used analgesic, acetaminophen (aspirin-free Anacin, Excedrin, Panadol, Tylenol) is usually not associated with side effects, though long-term use of acetaminophen is thought to be one of the common causes of end-stage renal disease.. To effectively control arthritis, aspirin must be taken in large, continuous doses (1000-5400 mg daily), which can cause stomach pain or damage; it is believed to cause more than 1,000 deaths annually in the United States. For that reason, some doctors prescribe one of several chemical variations referred to as nonacetylated salicylates, such as CMT, Tricosal, and Trilisate, which can cause deafness or ringing in the ears in large doses.
Much stronger analgesics are also prescribed for arthritis, sometimes along with acetaminophen. These are: codeine (Dolacet, Hydrocet, Lorcet, Lortab); morphine (Avinza, Oramorph); oxycodone (Vicodin, Oxycontin, Roxicodone); propoxyphene (Percocet, Darvon, Darvocet) and tramadol (Ultram, Ultracet). These medicines can cause psychological and physical dependence, as well as constipation, dizziness, lightheadedness, mood changes, nausea, sedation, shortness of breath and vomiting. Taking high doses or mixing with alcohol can slow down breathing, a potentially fatal condition.
Analgesics don’t treat the inflammation that can cause severe arthritis pain. For inflammation, steroids, NSAIDs and newer COX-2 inhibitors are prescribed. Corticosteroids (Cortisone), prednisone and related medications can cause bruising, cataracts, elevated blood sugar, hypertension, increased appetite, disease.. To effectively control arthritis, aspirin restlessness, osteoporosis, susceptibility to infection and thin skin.
Twenty NSAIDs are available with a doctor’s prescription, with three of those also available over the counter. They are diclofenac (Arthrotec, Cataflam, Voltaren); diflunisal (Dolobid);etodolac (Lodine); fenoprofen calcium (Nalfon); flurbiprofen (Ansaid); ibuprofen (Advil, Motrin IB, Nuprin); indomethacin (Indocin); ketoprofen (Orudis); meclofenamatesodium (Meclomen); mefenamic acid (Ponstel); meloxicam (Mobic); nabumetone(Relafen); naproxen (Naprosyn, Naprelan); naproxen sodium (Anaprox, Aleve);oxaprozin (Daypro); piroxicam (Feldene); sulindac (Clinoril); and olmetin sodium (Tolectin).
Side effects of NSAIDs include abdominal or stomach cramps, edema (swelling of the feet), pain or discomfort, diarrhea, dizziness, drowsiness or lightheadedness, headache, heartburn or indigestion, nausea or vomiting, gastric ulcers, stomach irritation, bleeding, fluid retention, and decreased kidney function. This is because NSAIDs act on arthritis by inhibiting prostaglandins, which protect the stomach lining, promote clotting of the blood, regulate salt and fluid balance, and maintain blood flow to the kidneys. The gastrointestinal complications of NSAIDS are the most commonly reported serious adverse drug reaction, though NSAIDs are reported to cause more than 10,000 deaths and 100,000 hospitalizations annually.
The newer group of arthritis drugs is known as cyclo-oxygenase-2 inhibitors (COX-2), which include Celebrex, Bextra and Vioxx. These medications have the same side effects as NSAIDS, except they are less likely to cause bleeding stomach ulcers and increase susceptibility to bruising or bleeding.
Non-selective NSAIDS have been associated with an increased risk of congestive heart failure. Less is known or has been concluded about the cardiovascular effects of COX-2 inhibitors, though a retrospective analysis of the risk of hospital admission for heart failure done by the Institute for Clinical Evaluative Sciences in Toronto, Canada suggests some may have serious side effects. The study of 130,000 older patients found that those using Vioxx had an 80% increased risk of hospital admission for congestive heart failure. Those using non-selective NSAIDS had a 40% increased risk, and those using Celebrex had the same rate of heart failure as people who had never used NSAIDS.
Antipyretic and anti-inflammatory effects of NSAIDs can mask the signs and symptoms of infection. Their use can interfere with the pharmacologic control of hypertension and cardiac failure in patients who take beta-adrenergic antagonists, angiotensin-converting enzyme inhibitors, or diuretics. Long-term use may damage chondrocyte (cartilage) function.
About 60% of patients will respond to any single NSAID. Approximately 10% of rheumatoid arthritis patients will not respond to any NSAID. Biologic response modifiers such as adalimumab (Humira); etanercept (Enbrel); infliximab (Remicade), and anakinra (Kineret)) are prescribed to either inhibit or the supplement the immune system components called cytokines.
Rare reports of lupus (with such symptoms as rash, fever and pleurisy) have been linked to treatment with adalimumab, etanercept and infliximab. Lupus symptoms resolve when the medication is stopped.
Multiple sclerosis has rarely developed in patients receiving biologic response modifiers. Seizures have been reported with etanercept.
Cannabis: By comparison, the side effects associated with cannabis are typically mild and are classified as “low risk.” Euphoric mood changes are among the most frequent side effects. Cannabinoids can exacerbate schizophrenic psychosis in predisposed persons. Cannabinoids impede cognitive and psychomotor performance, resulting in temporary impairment. Chronic use can lead to the development of tolerance. Tachycardia and hypotension are frequently documented as adverse events in the cardiovascular system. A few cases of myocardial ischemia have been reported in young and previously healthy patients. Inhaling the smoke of cannabis cigarettes induces side effects on the respiratory system. Cannabinoids are contraindicated for patients with a history of cardiac ischemia. In summary, a low risk profile is evident from the literature available. Serious complications are very rare and are not usually reported during the use of cannabinoids for medical indications.
Source: Americans for Safe Access