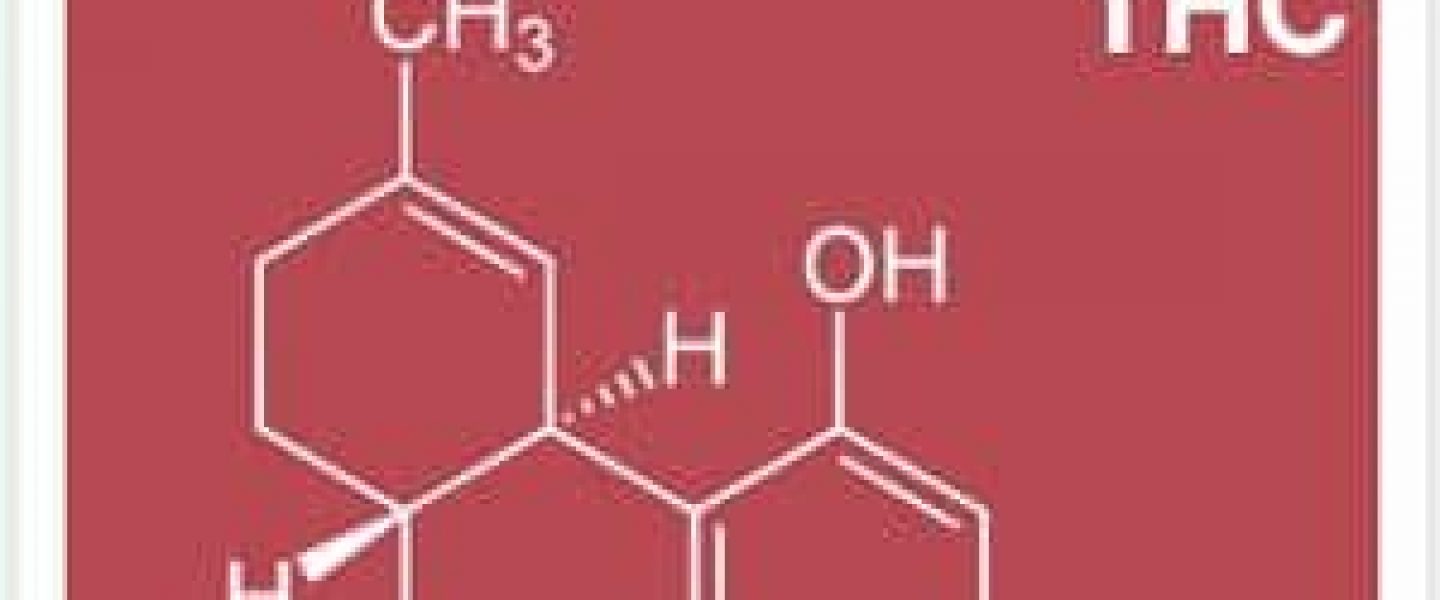
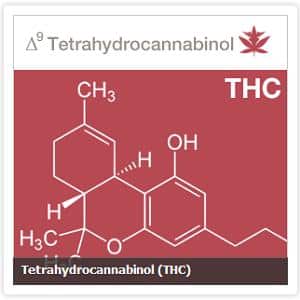
 Marijuana Science Will Finally Get It’s Rightful Day In Court
Marijuana Science Will Finally Get It’s Rightful Day In Court
By Paul Armentano, NORML Deputy Director
The U.S. Court of Appeals for the D.C. Circuit will hear opening arguments next week in a lawsuit challenging the federal government’s refusal to consider reclassifying cannabis as a schedule I prohibited substance under federal law.
At issue in the case is whether the Drug Enforcement Administration (DEA) acted appropriately when the agency last year denied an administrative petition - initially filed by a coalition of public interest organizations, including NORML, in 2002 – that called on the agency to initiate hearings to reassess the present classification of cannabis.
Under federal law, schedule I substances must possess three specific criteria: “a high potential for abuse;” “no currently accepted medical use in treatment;” and “a lack of accepted safety for the use of the drug … under medical supervision.” In its 2011 denial of petitioners’ rescheduling request, DEA Administrator Michele Leonhart alleged that cannabis possesses all three criteria, claiming: “[T]here are no adequate and well-controlled studies proving (marijuana’s) efficacy; the drug is not accepted by qualified experts. … At this time, the known risks of marijuana use have not been shown to be outweighed by specific benefits in well-controlled clinical trials that scientifically evaluate safety and efficacy.”
By contrast, a recent scientific review of clinical trials evaluating the safety and efficacy of cannabis concluded, “Based on evidence currently available the Schedule I classification is not tenable; it is not accurate that cannabis has no medical value, or that information on safety is lacking.”
Commenting on the upcoming hearing in a press release, Joe Elford, Chief Counsel with Americans for Safe Access (ASA) said: “Medical marijuana patients are finally getting their day in court. What’s at stake in this case is nothing less than our country’s scientific integrity and the imminent needs of millions of patients.” Elford will be arguing the case before the D.C. Circuit. Oral arguments in the case are scheduled for Monday, October 16th.
NORML previously filed a similar rescheduling petition with the DEA in 1972, but was not granted a federal hearing on the issue until 1986. In 1988, DEA Administrative Law Judge Francis Young ruled that marijuana did not meet the legal criteria of a Schedule I prohibited drug and should be reclassified. Then-DEA Administrator John Lawn rejected Young’s determination, a decision the D.C. Court of Appeals eventually affirmed in 1994.
A subsequent petition was filed by former NORML Director Jon Gettman in 1995, but was rejected by the DEA in 2001.
Further information on the lawsuit is available at: www.safeaccessnow.org. Additional information on the 2002 petition to reschedule cannabis is available at: www.drugscience.org.
Source: NORML