By Paul Armentano, NORML Deputy Director
By Paul Armentano, NORML Deputy Director
State Public Health Department officials have recommended over $7 million dollars in grant funding to pay for a series of state-sponsored clinical trials to assess the safety and efficacy of cannabis and cannabinoids.
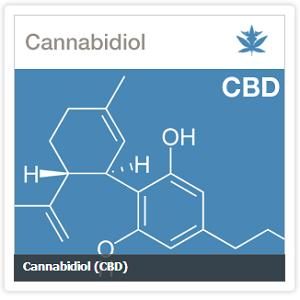
The proposed studies include a pair of clinical trials to evaluate the use of cannabidiol (CBD), a nonpsychotropic plant cannabinoid, for patients with pediatric epilepsy. Two additional trials will assess the use of cannabis for patients suffering from post-traumatic stress. Other studies will assess the efficacy of either cannabis or CBD in the treatment of Parkinson’s disease, brain tumors, ulcerative colitis, and pain management. (More specific summaries of all eight proposed studies are available online here.)
Grant funding for the proposed studies requires final approval by the state Board of Health in December.
Following funding approval, researchers will still be required to gain additional federal approval in order to obtain access to research-grade cannabis or CBD.
The state of California previously sponsored a similar series of clinical trials assessing the safety and efficacy of marijuana. Those studies evaluated the use of whole-plant cannabis in patients with neuropathy, multiple sclerosis, and autoimmune deficiencies. A summary of those trials, published in 2012, concluded, “Based on evidence currently available the Schedule I classification is not tenable; it is not accurate that cannabis has no medical value, or that information on safety is lacking.”
Source: NORML - make a donation