
There is a lot going on these days with CBD (Cannabidiol), especially after the passage of the Farm Bill on Nov. 29, 2018, essentially legalized hemp throughout the nation and by extension hemp-derived CBD.
CBD marketers, especially in the cosmetics industry, could not have been more delighted.
Suddenly CBD and its various miraculous benefits are being touted everywhere, including serious gushing about its benefits in The New York Times Sunday Style Section*.*
Jonathan Miller, general counsel for the US Hemp Roundtable, an industry trade group, recently claimed that: “CBD now is too big to fail.”
Now, federal agencies are getting on board, Quartz noted recently, and there is big money to be made:
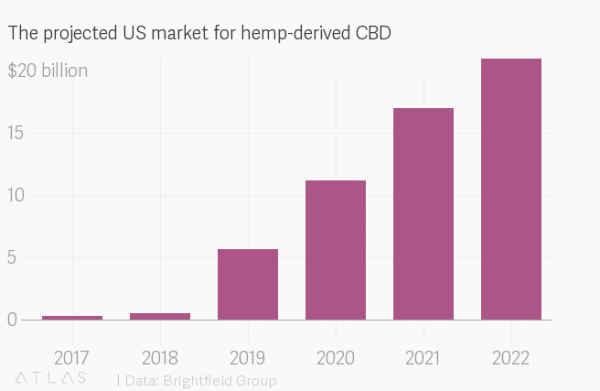
The Farm Bill served to remove CBD from its classification as a Schedule I drug and clarified its status as an agricultural product, ensuring that farmers cultivating it will be able to legally obtain crop insurance.
The same day of the bill’s passage, Food and Drug Administration commissioner Scott Gottlieb issued a statement reminding Americans that it maintained the FDA’s purview over food, drugs, cosmetics, and dietary supplements containing hemp-derived CBD, and announced the FDA’s intentions to regulate them.
Gottlieb, however, took issue with companies making unsubstantiated medical claims about CBD’s abilities to treat diseases such as cancer and diabetes, casting doubt over the effectiveness of the plant’s benefits.
A New York Times article from Dec. 26, 2018 noted that a National Academies of Sciences, Engineering and Medicine panel “concluded that there was insufficient evidence that CBD was effective in treating conditions like insomnia, addiction to cigarettes and Parkinson’s disease, and limited evidence in its ability to treat anxiety.”
The NY Times did concede that there is far too little data and research on CBD precisely because it has been classified as a drug with little medical value all these years by the DEA.
None of this seemed to bother hemp’s general counsel, Jonathan Miller, who sees it all as good news, including oversight from the FDA.
“We want the FDA to be going after the bad guys in the industry who don’t want to play by the rules…The problem our industry has—and is going to have—is with people in our industry that make false claims and that sell products that aren’t what they say they are,” Miller told Quartz.





